
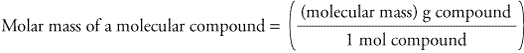
This is the same as the number of sodium ions in 1 mol of sodium chloride. For example, the number of atoms in 1 mol of sulfur is the same as the number of molecules in 1 mol of sulfur dioxide. The amount in moles can apply to atoms, molecules, ions and electrons. The number of atoms, molecules or ions in one mole of a substance is called the Avogadro constant. The particles can be atoms, molecules or ions. One mole of a substance contains the same number of particles as one mole of any other substance. The table shows the masses of one mole of three substances. Formula Calculate Formatted Formula H 2 O Empirical Formula H 2 O Molar Mass 18.02. the relative formula mass ( M r ) of its formula in grams if the substance is a compound Molar Mass of Water Molecular Weight of H 2 O.the relative atomic mass ( A r ) of its formula in grams if the substance is an element.The mass of one mole of a substance is equal to: For this reason, chemical amounts are measured in moles. The actual masses of atoms, molecules and ions are too small to be useful in calculations. Mass of 3.9 × 10 25 molecules of H 2 O = 1167 g.Īnswer: the mass of 3.9 × 10 25 molecules of H 2 O is 1167 g.Introducing the mole, the unit of measurement for the number of particles in a substance The question is asking about the mass of 3.9 × 10 25 molecules of water.
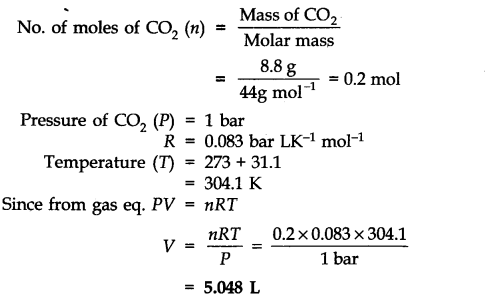
So the molar mass of H 2 O = 2(Molar mass of hydrogen atoms) + 1(Molar mass of oxygen atoms). We know that there are two hydrogen atoms and one oxygen atom for every H 2 0 molecule. To find the mass of 3.9 × 10 25 H 2 O molecules, first we have to find the mass of one molecule of H 2 O. Q.3 Calculate the mass in grams of 3.9 × 1025 H2O molecules Step 1 Mass of 9.25 10 22 molecules of H 2 O = 2.768 g.Īnswer: the mass of 9.25 10 22 molecules of water is 2.768 g. The question is asking about the mass of 9.25 10 22 molecules of water. Mass of one mole of H 2 0 (Water) = 18.02 g So, the molar mass of H 2 O = 2(Molar mass of hydrogen atoms) + 1(Molar mass of oxygen atoms) To find the mass of 9.25 10 22 molecules of water, firstl we have to find the mass of one molecule of water (H 2 O). Q.2 What is the mass of 9.25 1022 molecules of water? Step 1 The question is asking about the mass of 2.5 × 10 9 molecules of water. Molar mass of H 2 0 =18.02 g/mol Mass of one mole of H 2 0 (Water) = 18.02 g So, the molar mass of H 2 O = 2(Molar mass of hydrogen atoms) + 1(Molar mass of oxygen atoms). To find the mass of 2.5 × 10 9 H 2 O molecules, first we have to find the mass of one molecule of H 2 O. More examples Q.1 Calculate the mass in grams of 2.5 × 109 H2O molecules Step 1 Mass of 1.00×10 24 molecules of H 2 O = 29.9 g.Īnswer: the mass of 1.00×10 24 molecules of water is 29.9 g. The question is asking about the mass of 1.00×10 24 (a septillion) molecules of water. Mass of X molecules of H 2 O = (Mass of a mole of H 2 O molecules) (X molecules)/ 6.022 x 10 23 Mass of X molecules of H 2 O / X molecules = Mass of a mole of H 2 O molecules / 6.022 x 10 23 This relation is then used to ‘convert’ a number of H 2 O molecules to grams by the ratio:
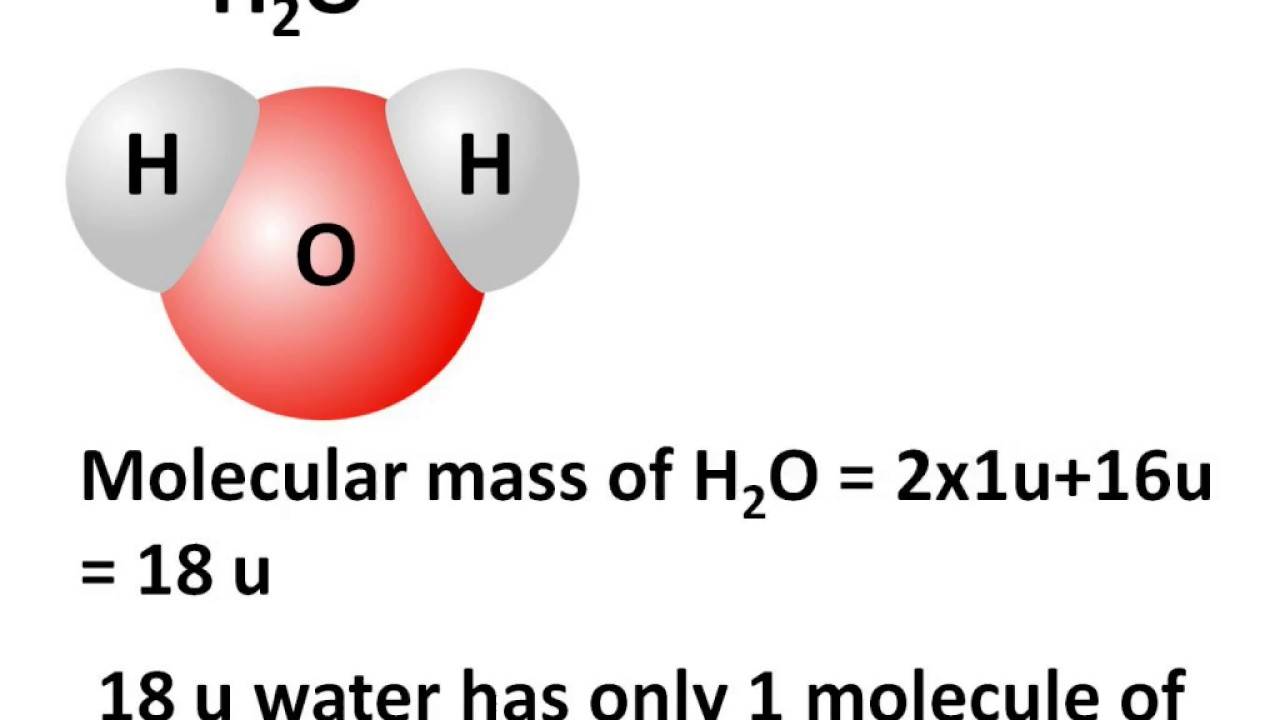
One mole of H 2 O is 6.022 x 10 23 molecules of H 2 O (Avogadro’s number). Molar mass of H 2 0 =18.02 g/mol Mass of one mole of H 2 0 (Water) = 18.02 g Step 2 So, the molar mass of H 2 O = 2 (Molar mass of hydrogen atoms) + 1(Molar mass of oxygen atoms).


 0 kommentar(er)
0 kommentar(er)
